Acidified Potassium Dichromate
The electron-half-equation for this reaction is. 04 g solid NaOH and dilute to 1000 ml.

The Oxidation Of Alcohols Chemistry Libretexts

Oxidation Of Aniline With Acidified Potassium Dichromate Gives
Why Wont The Compound React With Acidified Potassium Dichromate The Student Room
Product V is the major product.

Acidified potassium dichromate. It is an ionic compound with two potassium ions K and the negatively charged dichromate ion Cr2O7- in which two hexavalent chromium atoms with oxidation state 6 are each attached to three oxygen atoms as well. In this reaction chromium will be reduced from an oxidation state of 6 in Cr 2 O 7 2-to 3 in Cr 3. It is a crystalline ionic solid with a very bright red-orange color.
Potassium dichromate and hydrogen peroxide reaction in acidic medium. A neutral organic compound X of molecular formula C2H6O on oxidation with acidified potassium dichromate gives an acidic compound Y. As with all hexavalent chromium compounds it is acutely and chronically harmful to health.
Heat S in a test tube. K 2 Cr 2 O 7 H 2 SO 4 3SO 2 K 2 SO 4 Cr 2 SO 4 3 H 2 O Chromium sulphate green b An aqueous solution or sodium carbonate extract of the salt produces a O Barium. Sulphur dioxide gas SO 2 is produced.
R is a sulphate salt. A redblue oscillating reaction. If oxidation occurs the orange solution containing the dichromateVI ions is reduced to a green solution containing chromiumIII ions.
It forms dense white fumes with concentrated hydrochloric acid. Aldehydes but not ketones can be oxidized easily to carboxylic acid with acidified potassium dichromate K2Cr2O7 H as oxidizing agent. 75 What is the trade name for fluoride ore of calcium.
H 2 SO 4 green. The gas turns potassium dichromate paper acidified with dil. CH 3 CH 2 OH 2O CH 3 COOH H 2 O.
Use this practical to investigate the oxidation reactions of various alcohols with acidified potassium dichromate. The oxidising agent used in these reactions is normally a solution of sodium or potassium dichromateVI acidified with dilute sulphuric acid. 전체 반응과정은 아래와 같다.
Reaction with acidified potassium dichromate solution A no reaction no reaction B no reaction orange to green C decolourises orange to green D decolourises no reaction 13. The potassium dichromate solution must be acidified by adding concentrated sulfuric acid. 74 Consider a titration of potassium dichromate solution with acidified Mohrs salt solution using diphenylamine as an indicator.
This will make a 010 N solution. RCHO O -- RCOHO Reducing agents can reduce an aldehyde to a primary alcohol and a ketone to a secondary alcohol. If oxidation occurs the orange solution containing the dichromateVI ions is reduced to a green solution containing chromiumIII ions.
The resulting oxalic acid solution is then used to titrate MnO 4 - to the endpoint of the titration which is the point at which the last drop of MnO 4 - ion is decolorized and a faint pink color persists for 30 seconds. It turns damp red litmus paper blue. H2SO4 to give a sweet smelling substance Z.
Compound X reacts with Y on warming in the presence of conc. Potassium dichromate K 2 Cr 2 O 7 is a common inorganic chemical reagent most commonly used as an oxidizing agent in various laboratory and industrial applications. X on heating gives brown coloured solid Y and two pungent gases A and B.
K 2 Cr 2 O 7 H 2 SO 4 3SO 2 K 2 SO 4 Cr 2 SO 4 3 H 2 O Chromium sulphate green b An aqueous solution or sodium carbonate extract of the salt produces a. Identify the gasgases given off. The oxidising agent used in these reactions is normally a solution of sodium or potassium dichromateVI acidified with dilute sulphuric acid.
The solution will become green in colour due to formation of Cr_ 2 SO_ 4 _ 3. It turns orange acidified potassium dichromateVI green. Includes kit list and safety instructions.
B Pass the gas through acidified potassium dichromate solution orange in colour. The gas turns potassium dichromate paper acidified with dil. A colourless pungent gas is evolved.
Reacts with acidified potassium dichromate solution Cr 2 O 7 2 H to form Product T which does not react with Benedicts reagent reacts with H 2 O H to form two products U and V. A turns acidified potassium dichromate solution green. 표본에서의 에탄올의 농도는 acidified potassium dichromate을 가지고 역적정을 통해 알아낼 수 있다.
Acidified orange colour potassium dichromate solution is oxidized to green colour chromium salt while hydrogen peroxide is oxidized to oxygen gas. K 2 Cr 2 O 7 3H 2 O 2 4H 2 SO 4 Cr 2 SO 4 3 3O 2 K 2 SO 4 7H 2 O. 충분한 양의 다이크로뮴산 칼륨과 표본의 반응에서 에탄올은 모두 아세트산으로 산화된다.
We have a Kickstart employment and training opportunity within our Early Years and Primary Education team as an Education Resource Assistant. Standardize with potassium bi-iodate or potassium dichromate before use. The electron-half-equation for this reaction is as follows.
H 2 SO 4 green. In association with Nuffield Foundation. The structure of isoprene is A H 3C CH CH 3 CH 2 C OH O B H 2C C CH CH 2 CH 3 C H 2C CH CH 2 D.
The oxidizing agent used in these reactions is normally a solution of sodium or potassium dichromateVI acidified with dilute sulfuric acid. Makes 0025N Na 22 S O 3 5H 2 O 6 Potassium dichromate Dissolve 4903 g K 22 Cr O 7 in distilled water and bring up to 1 liter. Acidified K _ 2 Cr_ 2 O_ 7 is oxidising agent whereas SO_ 2 is reducing agent.
The net effect is. 479 recall that aldehydes and ketones can be distinguished using acidified potassium dichromateVI Fehlings solution and Tollens reagent with Fehlings solution and Tollens reagent viewed as Cu² and Ag respectively. The chemical formula for potassium dichromate is K 2 Cr 2 O 7 and the molar mass is calculated to be 294185 gmol.
This reaction was once used in an alcohol breath test. Education Resource Assistant Note - you must apply for this post via your local jobcentre using the following reference. The oxidizing agent used in these reactions is normally a solution of sodium or potassium dichromateVI acidified with dilute sulfuric acid.
If oxidation occurs then the orange solution containing the dichromateVI ions is reduced to a green solution containing chromiumIII ions. For more information click on the link that follows. It is a redox reaction.
Potassium Dichromate Formula and Molecular Structure. Use this to standardize the 01 N sodium thiosulfate see. A sample of reagent grade sodium oxalate Na 2 C 2 O 4 is weighed out dissolved in distilled water acidified with sulfuric acid and then stirred until the oxalate dissolves.
Based on the information above draw the structural formulae of Unknown S and Products T U and V. The salt is popular in the laboratory because it is not. Click hereto get an answer to your question A compound X green coloured solid gets oxidised to reddish brown solid in presence of air.
Potassium dichromate K 2 Cr 2 O 7 is an oxidising agent it causes something else the ethanol to be oxidised. If oxidation occurs the orange solution containing the dichromateVI ions is reduced to a green solution containing chromiumIII ions. Sulphate ion SO 4 2-is present.
The number of moles of Mohrs salt required per mole of dichromate is a 3 b 4 c 5 d 6 Answer. A secondary alcohol can be oxidised into a ketone using acidified potassium dichromate and heating under refluxThe orange-red dichromate ion Cr 2 O 7 2 is reduced to the green Cr 3 ion.
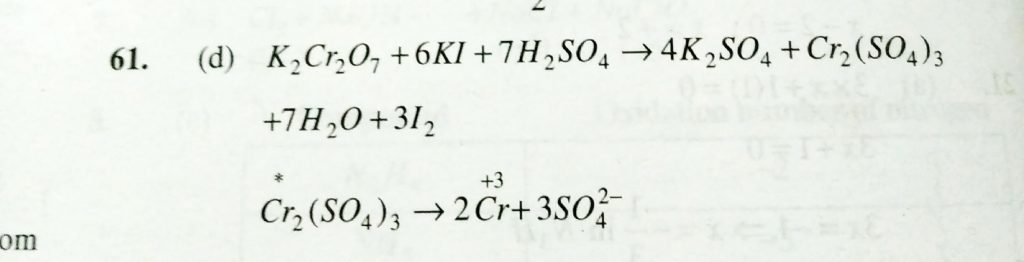
The Oxidation State Of Chromium In The Final Product Formed By The Reaction Between Ki And Acidified Potassium Dichromate Solution Is Sahay Lms

Ethanol On Reaction Of Acidified Potassium Dichromate Class 12 Chemistry Cbse
Canvas Bham Ac Uk

Production Of Aldehydes Ketones Cie A Level Chemistry Revision Notes

Oxidation Of Alcohols Compound Q

Potassium Dichromate Wikipedia
Chemistry Organic Naming Molecules Alcohols
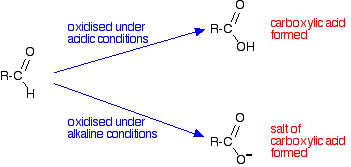
Oxidation Of Aldehydes And Ketones
