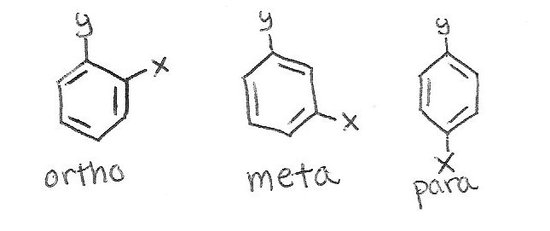
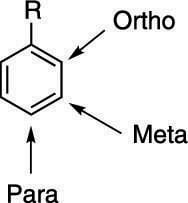
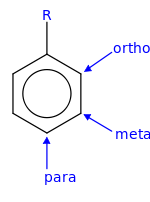
Benzene Ring Positions Ortho Meta Para
Although this o- m- p- system is the common naming system for benzene derivatives they have been applied broadly in books and literatures. Thus toluene reacts faster than benzene at the ortho and para positions.

Ortho Para Meta In Eas With Practice Problems Chemistry Steps
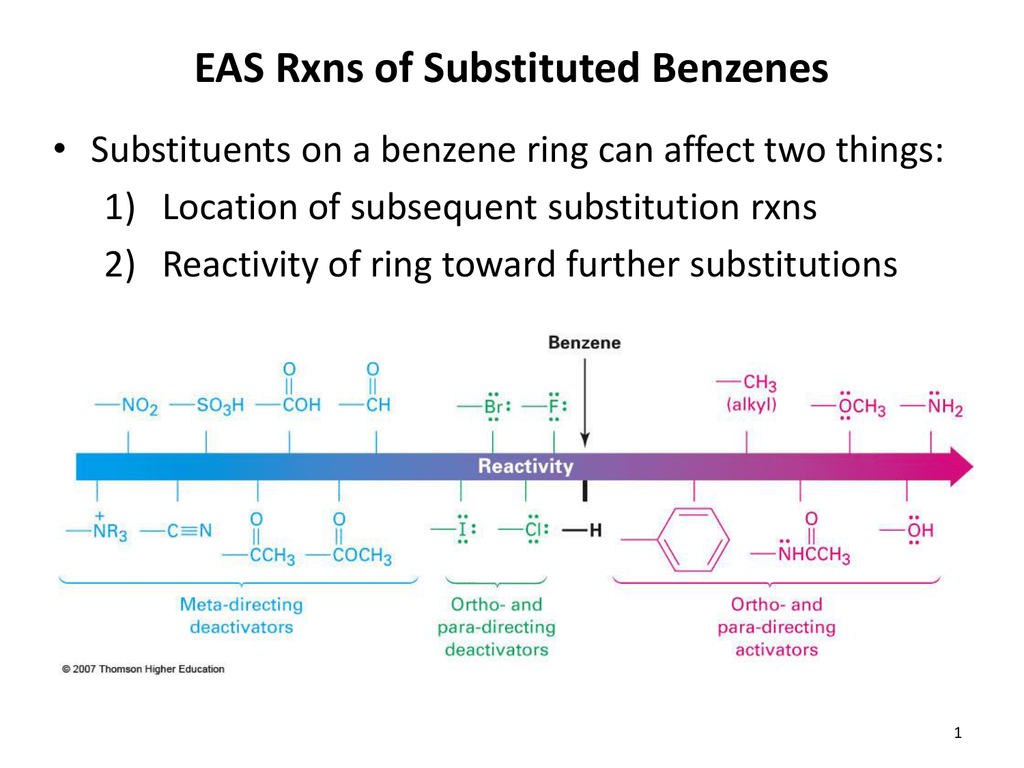
Ortho Para Directing Groups

Ortho Para Meta In Eas With Practice Problems Chemistry Steps
Each benzene ring H-atom is given a standard shift value of 736 ppm and adjusted by up to 5.

Benzene ring positions ortho meta para. 12- next to each other in a benzene ring. For di-substituted benzene there is another unique way to indicate the relative position of the two substituents by using ortho- meta- and para-. Or para abbreviated o- m- or p-.
Meta definition pertaining to or noting a story conversation character etc that consciously references or comments upon its own subject or features often in the form of parody. So we can ignore it but we need to understand how the proton hydrogen shows up in the ortho and meta positions. Para definition a coin and monetary unit of Macedonia and Serbia one 100th of a dinar.
In para-substitution the substituents occupy the opposite ends positions 1 and 4 corresponding to R and para in the. The small letters o- m- and p- standing for ortho- meta- and para- preceding the name xylene are used to identify the three different isomers that vary in the ways the two methyl groups displace the hydrogen atoms of benzene. Y Trichloromethyl benzene In presence of F e C l 3 and in dark electrophilic substitution on the benzene ring by C l ion at ortho and para positions occurs to give o- and p- chlorotoluene.
Chemistry usually in italics denoting that an organic compound contains a benzene ring with substituents attached to atoms that are directly opposite across the ring the 14- positions. Because it contains only carbon and hydrogen atoms benzene is classed as a hydrocarbon. Other functional groupssuch as COOH NO 2 and SO 3Hare electron withdrawing deactivating the ring and instead direct toward the meta positions McMurry 561.
Ortho J meta or ddd J ortho J ortho J meta H c td J ortho J meta or ddd J ortho J ortho J meta H d dd J ortho J. Disubstituted benzene rings can be named based on the relative positions of the substituents. In meta-substitution the substituents occupy positions 1 and 3 corresponding to R and meta in the diagram.
The para configuration actually has two planes of symmetry one vertical and one horizontal on the structure below through the benzene ring which only allows two distinct aryl absorptions to occur. The closer the proton is to the other hydrogen atoms the greater the effect on the proton. Ortho meta para ortho para meta 44 - 55 99 trace 70 - 30 100 trace 37 1 62 99 1 18 80 2 20 80 19 80 1 20 80 64 932 03 67 932 Substituent CH 3 58 4 38 96 4 Organic Lecture Series 28 Orientation.
Ortho meta and para are not used in systematic nomenclaure CH 3 2-ch lorotoluene ortho-chlorotoluene o-chlorotoluene H CH 3 13-dim ethylbnz meta-xylene m -xylene CO2H Cl 4- ch. Disubstituted benzene rings can be named based on the relative positions of the substituents. If a substituted benzene undergoes further substitution mostly at the ortho and para positions the original substituent is called an ortho para-directing group.
The hydrogen at the para-position of the benzene ring is unaffected by coupling. Para p- Y X Y X Y X Note. That the ortho and para positions will be more shielded but does not indicate which one will be.
Due to its various resonating structures theres an excess of an electron or negative charge over ortho- and para- positions of the ring than the meta- position. When there are two substituents on the benzene ring numbers can be used to identify the position of the substituents but an older system of nomenclature is often used instead in which the prefixes ortho- meta- and para- often abbreviated as o- m- and p- are used to. The meta configurations plane of symmetry mirrors two carbons of the benzene ring allowing for four aryl absorptions to occur.
The origin of this. It is its common name and also an accepted IUPAC name. The terms ortho meta and para are prefixes used in organic chemistry to indicate the position of non-hydrogen substituents on a hydrocarbon ring benzene derivative.
In ortho-substitution two substituents occupy positions next to each other which may be numbered 1 and 2In the diagram these positions are marked R and ortho. When reaction of toluene occurs at the meta position then the resonance forms of the sigma complex put positive charge over 3 secondary carbons - the same as for benzene. Due to I effect electron withdrawing nature of halogen benzene ring gets somewhat deactivated towards electrophilic substitution reaction.
A movie about making a movie is just so metaespecially when the actors criticize the acting. Benzene is a natural constituent of crude oil and is one of the elementary petrochemicals. Certain substituents direct preferentially to ortho.
Relative position of the substitutents 12-disubstituted. As structure of phenol involves a benzene ring in its substituted compounds the terms ortho 12- disubstituted meta 13-disubstituted and para 14-disubstituted are often used in the common names. Ortho meta and para historically carried different meanings but in 1879 the American Chemical Society settled upon.
The prefixes derive from Greek words meaning correctstraight followingafter and similar respectively. The prefix ortho is used if the substituents occupy adjacent positions on the ring 12 meta is used if the substituents are separated by one ring position 13 and para if they are found on opposite sides of. Ortho- Meta- Para- OMP Nomenclature for Disubstituted Benzenes.
Is named by prefixing the alkyl group name to the word benzene. Ring for further substitutions and direct subsequent reactions to the ortho- and para- positions. Thus bromine is an ortho para-directing group because all electrophilic substitution reactions of.
Since methyl group activates the ring at ortho and para positions more than meta positions the electroscopic substitution occurs mostly at these positions. Ortho-Couplings Have a High J-Value. On a mono-substituted benzene ring containing an activating group the new electrophile will add to the ortho and the para positions of the ring figure 7a.
Others to meta positions substituents are classified as either. Instead of using numbers to indicate substituents on a benzene ring ortho- o- meta- m- or para p- can be used in place of positional markers when there are two substituents on the benzene ring disubstituted benzenes. The simplest hydroxy derivative of benzene is phenol.
An example of this is named methylbenzene or toluene If there are only two groups attached to the benzene ring their relative positions can be designated by numbers or by the terms ortho meta. They are defined as the following. In the case of most deactivating groups on the ring the electrophile will add to the meta position figure 7b.
The prefix ortho is used if the substituents occupy adjacent positions on the ring 12 meta is used if the substituents are separated by one ring position 13 and para if they are found on opposite sides of. Benzene is an organic chemical compound with the molecular formula C 6 H 6The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each.
Where Are The Ortho Meta And Para Positions Located Quora

Org Chem Text Chapter 5 5 8 Htm

How To Identify Ortho Para Meta Positions In A Benzene Ring Chemistry Alcohols Phenols And Ethers 9249031 Meritnation Com

Ortho Para Meta Positions Example Of Group Showing Ortho Para Meta Positions
Inductive Effects Of Alkyl Groups Chemistry Libretexts
How Electron Withdrawing Substituents Direct The Substitution On A Benzene Ring Dummies
Nomenclature Of Aromatic Compounds Chemgapedia
Arene Substitution Pattern Wikipedia