Calcium Carbonate Acetic Acid
The main target users are workers and those responsible for occupational safety and health. Visit BYJUS for more content.

Effect Of Acetic Acid In The Calcium Carbonate Content Of Chicken Download Scientific Diagram
Seashell And Vinegar Experiment Wairoa Public Library Facebook
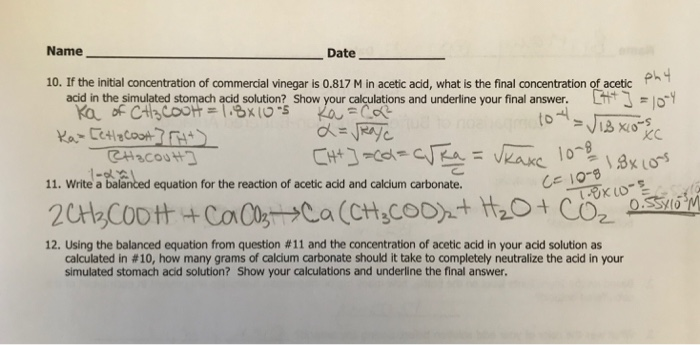
Solved Name Date 10 If The Initial Concentration Of Chegg Com
Water is NOT produced from this reaction--vinegar has water in it as in diluted to 5-10 acidity.

Calcium carbonate acetic acid. Calcium Chloride CaCO3 Calcium Carbonate CaF2 Calcium Fluoride CaH2 Calcium Hydride CaI2 Calcium Diiodide CaO Calcium Oxide CaS Calcium Sulfide CaSO4 Calcium Sulfate CBr4. Dolostone is a rock composed of almost entirely of dolomite. The latter depends on the acid concentration since it needs two molecules of HCl to convert one molecule of calcium carbonate into calcium chloride.
Indeed the versatility of water as a solvent is essential to living organisms. Its important to remember that common names are inaccurate and vary from one place and time to another. At that time Germany was producing 10000 tons of glacial acetic acid around 30 of which was used for the manufacture of indigo dye.
Limestone is composed almost entirely of calcite and will produce a vigorous fizz with a drop of hydrochloric acid. The acetic acid was isolated by treatment with milk of lime and the resulting calcium acetate was then acidified with sulfuric acid to recover acetic acid. This is the list of all compounds with density tables present in the full database - it was actual on May 4 th 2005 as the list is growing it may be already incomplete.
May want to let evaporate to remove any remaining vinegar and then add your own water. If Tums is added to 350 mL of 0300 M. On reacting NaHCO 3 and acetic acid the gas evolved turns lime water milky.
Formula Type Chemical Name CaO I Calcium oxide C 2 H 2 M Dicarbon dihydride LiOH I Lithium hydroxide SO 3 M Sulfur trioxide. A Amyl CAB B D ABenzyl ABAC DD AButyl AB B AC D 2Diacetone AAC DD AEthyl ABAC AHexyl AAC D B AIsobutyl ABAC. I the presence of catalase ii the oxidation and overoxidation of ethanol to acetic acid and to carbon dioxide and water respectively iii oxidation of lactate to carbonate iv oxidation of glycerol to dihydroxyacetone and v acid production from d-glucose.
It will produce a very weak fizz when a drop of cold hydrochloric. To Learn about the structure of Acetic acid its preparations chemical physical properties uses and FAQs. Calcium carbonate is a chemical compound with the formula Ca CO 3It is a common substance found in rocks as the minerals calcite and aragonite most notably as limestone which is a type of sedimentary rock consisting mainly of calcite and is the main component of eggshells snail shells seashells and pearlsCalcium carbonate is the active ingredient in agricultural lime and is created when.
The vinegar acetic acid reacts with the eggshell calcium carbonate to produce a water-soluable compound calcium acetate and carbon dioxide gas the bubbles on the eggshell. Different values of the pure water 0 concentration density reflect the fact that the measurements were done in different temperatures. This would be called neutralisation if you take the equivalent amount of both components.
If there is a multi-valent metal present write both the stock and classical name. A i and ii Reaction between Sodium hydrogen carbonate and acetic acid leads to the evolution of carbon-dioxide gas. Acetic Acid CH3COOH- Acetic Acid is an organic compound with formula CH3COOHVinegar is a water solution of acetic acid containing 5-8 of acetic acid by volume.
Check our FAQ section for more details. Answer 1 of 5. As a result it forms a protective layer around the limestone preventing further access by the acid and effectively stopping the reaction.
So youd get water carbon dioxide and calcium acetate a water soluble salt. Water a substance composed of the chemical elements hydrogen and oxygen and existing in gaseous liquid and solid states. Sulphuric acid is a very strong acid but it wont react very well with calcium carbonate because one product of the reaction is calcium sulphate.
Calcium Carbonate is the carbonic salt of calcium CaCO3. Calcium carbonate CaCO3 reacts with stomach acid HCl hydrochloric acid according to the following equation. On reacting NaHCO 3 and acetic acid carbon dioxide gas is evolved along with formation of sodium ethanoate.
Acetic Acid Glacia1 BA A CD B D Acetic Acid 20 BA A CD C Acetic Acid 80 BA AC DB C Acetic Acid B AA CDB C Acetic Anhydride B AA CD D Acetone6 A BA D DCB Acetyl Chloride C AD A Acetylene2 AB BD A C Acrylonitrile AC B B DA C D Alcohols. Acetic acid acetic aldehyde acetone. Calcium Acetate is a calcium salt of acetic acid.
The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes. CO 2 turns the lime water milky and extinguish a burning splinter.
Acetic acid glacial 100 CAS 64-19-7 anhydrous for analysis EMSURE ACSISOReag. In order to have a complete reaction acid intake should be equal to the stoichiometric amount of minerals or even greater. If quantities are not equivalent this means at the end the medium remains acidic or alkaline you would have an.
Calcium acetate is administered orally to prevent or treat calcium deficiency and to treat hyperphosphatemia due to its phosphate-binding properties. Calcium is a mineral essential for many cellular functions including nerve impulse transmission muscle contraction cardiac function bone formation and capillary and cell membrane permeability. In conclusion pH condition affects the formation of polymorphs of calcium carbonate by shifting the equilibrium concentration of coronate species eg HCO 3 C O 3 2 or changing the CaCO 3 binding strength but other operating factors such as temperature CO 2 flow rate presence of acid and bases and carbonation time also appear to more significantly influence the.
This is plaster of paris and is insoluble. It has a pungent smell and a sour taste. Even so you rarely ask someone to pass the sodium chloride at the dinner table.
Ph Eur - Find MSDS or SDS a COA data sheets and more information. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses. Acetaldehyde S O Barium carbonate saturated S S Acetic acid 1-10 S S Barium carbonate saturated S S Acetic acid 10-60 S O Barium hydroxide S S Acetic acid 80-100 S O Barium sulfate saturated S S Acetic anhydride S S Barium sulfite saturated S S Acetone S S Beer S S.
My guess is that its calcium carbonate in the egg. CaCO3 2CH3COOH CaCH3COO2 H2O CO2. Chemical or scientific names are used to give an accurate description of a substances composition.
Calcium carbonate is used therapeutically as a phosphate buffer in hemodialysis as an antacid in gastric hyperacidity for temporary relief of indigestion and heartburn and as a calcium supplement for. The Acid Test on Rocks. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
The reaction of those is. It is one of the most plentiful and essential of compoundsA tasteless and odourless liquid at room temperature it has the important ability to dissolve many other substances. CaCO3s2HClaq-CaCl2aqH2OlCO2g Tums an antacid contains CaCO3.
The ICSC project is a common undertaking between the World Health Organization WHO and. Frateur in 1950 proposed a scheme for the classification of Acetobacter that was based on five biochemical criteria. Acetic Acid CH4 Methane CH4O Methanol CHCl3 Chloroform Cl2 Chlorine Gas ClO2 Chlorine Dioxide CoNO32 CobaltII Nitrite CO2 Carbon Dioxide.
Some rocks contain carbonate minerals and the acid test can be used to help identify them. LIMESTONE DOLOSTONE AND MARBLE. Answer Key Ionic Molecular or an Acid Honors Chemistry Write which type of compound it is whether the compound is ionic molecular or an acid.
This is one mole of potassium carbonate and two moles of hydrochloric acid. Vinegar is acetic acid.

Chemical And Physical Changes
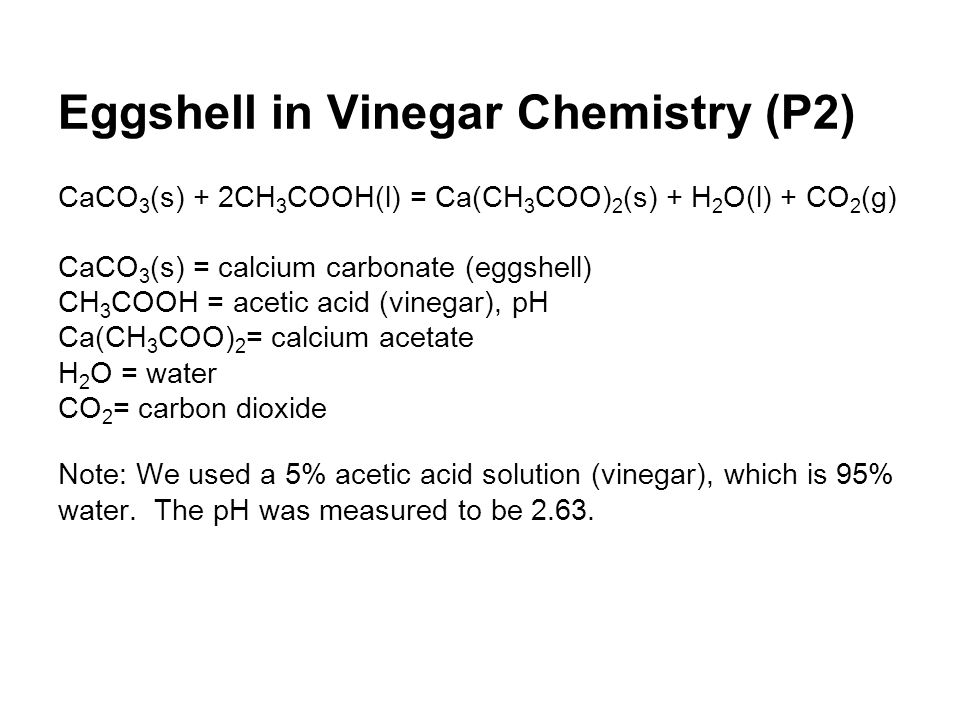
Eggsperiment Ppt Video Online Download

Changing States Science Experiment Abakcus

Rabeprazole Autoszakerto Magyarorszagon
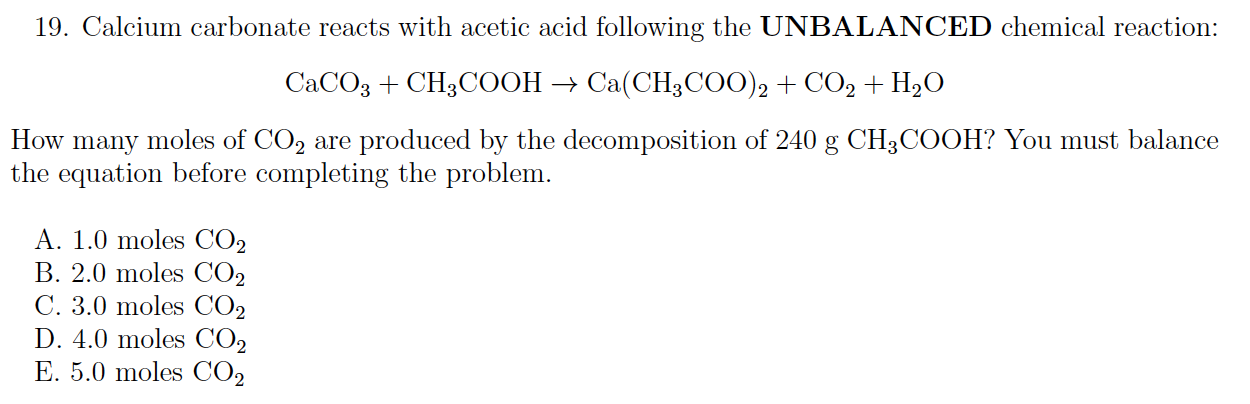
Solved 19 Calcium Carbonate Reacts With Acetic Acid Chegg Com
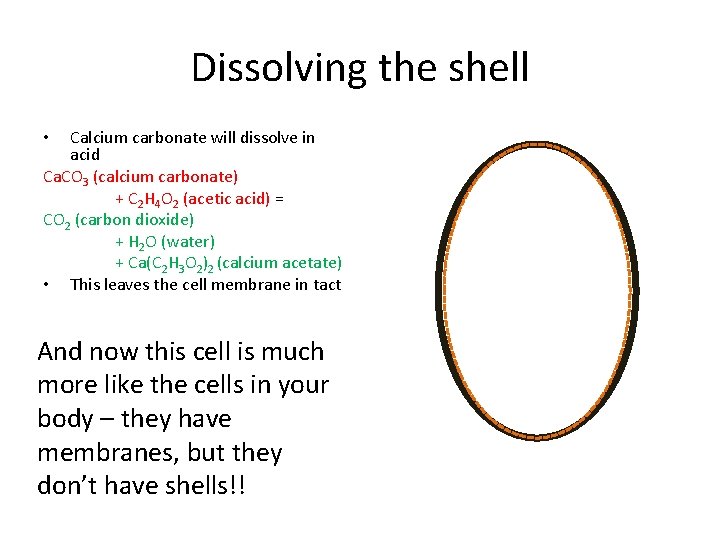
The Eggsperiment A Chicken Egg Is One Cell

The Homestead Laboratory Eggshell Calcium Carbonate Leavening Part 1

How To Balance Ch3cooh Caco3 Ch3coo 2ca Co2 H2o Youtube

